Présentation
Une base de données de produits naturels destinée au criblage de composés et à d’autres applications pour la recherche de composés naturels
Initialement développée par le Dr. Hartmut Laatsch, la bibliothèque Antibase bien connue est une base de données complète de produits naturels comprenant plus de 105 300 composés. Et nous avons ajouté plus de 9 500 composés dans notre dernière version!
Largement citée dans la littérature scientifique, cette base de données en pleine expansion est un outil efficace de criblage pour la métabolomique et la découverte de médicaments, des domaines dans lesquels les produits naturels et leurs dérivés peuvent accélérer l’avancée des traitements contre les maladies, notamment le cancer ou le diabète, ainsi que des thérapies antimicrobienne ou antifongique.
Utilisez le logiciel ChemWindow de Wiley pour effectuer des recherches en toute sécurité dans la collection, ainsi que des outils puissants pour créer vos propres bases de données, dessiner des structures, etc.
Une couverture complète. |
Des données soigneusement sélectionnées. |
De puissants outils d’exploration de données. |
|
Des découvertes plus rapides grâce à une couverture étendue des composés sur diverses catégories de cette riche ressource destinée à la recherche de produits naturels. |
Chez Wiley, nous priorisons la qualité des données pour fournir des résultats sur lesquels vous pouvez compter afin de prendre des décisions éclairées. |
Des outils d’exploration de données robustes pour obtenir des données plus détaillées, agréger et intégrer vos propres données, et plus encore ! |
Applications:
- Intégrez ces outils aux flux de travail de criblage à haut débit (HTS) et de déréplication dans l’ industrie pharmaceutique et la biotechnologie afin d’identifier rapidement les composés déjà étudiés et ainsi rationaliser la recherche de nouveaux composés ayant des effets antimicrobiens ou souhaités.
- Ces outils peuvent être utilisés dans divers secteurs tels que les industries agroalimentaire et cosmétique, l’agriculture, les pesticides et d’autres applications qui recourent principalement aux produits naturels ou dans le cadre de programmes universitaires et de recherche.
Quelles sont les nouveautés de la version 2025 ?
Nous avons augmenté la collection de 10 % avec l’ajout de plus de 9 500 nouveaux composés, y compris des composés de la dernière version de Natural Product Atlas.
Des données fiables provenant de sources dignes de confiance
Wiley fait autorité dans le domaine des données scientifiques. Nos bases de données reconnues sont traitées conformément aux protocoles rigoureux pour garantir leur plus grande qualité. Les procédures de qualification commencent dès l’acquisition des données et continuent durant tout le processus de développement des bases de données. Toute donnée obtenue de partenaires de confiance est soigneusement étudiée avant d'être incluse dans nos collections.
Principales fonctionnalités
| Des données soigneusement sélectionnées et vérifiées | Les données sont collectées à partir d’articles de recherche primaire et secondaire, puis soigneusement vérifiées et validées à l’aide de références clés et de sources fournies sous forme de champ de données. |
| Des métadonnées précieuses pour vous aider à affiner la recherche |
En plus de la structure chimique, d’autres métadonnées utiles ont été inclues pour chaque entrée:
|
| Données spectrales pour permettre la caractérisation |
|
| Des outils puissants pour l’exploration des données et la création de bases de données |
Comprend le logiciel ChemWindow de Wiley pour effectuer des recherches dans la collection, ainsi que des outils puissants pour créer vos propres bases de données, dessiner des structures, etc. |
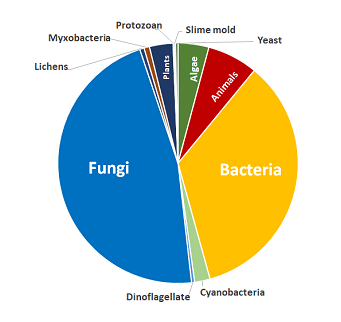
Composés couverts
Inclut des composés provenant des sources biologiques suivantes :
- Algues
- Animaux
- Bactéries
- Cyanobactéries
- Dinoflagellés
- Champignons
- Lichens
- Myxobactéries
- Plantes
- Protozoaires
- Myxomycètes
- Levures
Spécifications des bases de données
|
|
|
Compatibilité
- L’identificateur de produits naturels de Wiley est compatible avec le logiciel KnowItAll ChemWindow Edition.
- Il est également compatible avec d’autres éditions du logiciel KnowItAll que vous utilisez.
Informations de commande
|
Code Produit |
Produit |
|
978EALDB04188 |
Guide d’identification Wiley des produits naturels (Bibliothèque AntiBase + ChemWindow) (Wiley Identifier of Natural Products (AntiBase Library + ChemWindow Software)) (Abonnement d’un an) |
|
978EALDB05529 |
Guide d’identification Wiley des produits naturels (Bibliothèque AntiBase + ChemWindow) (Wiley Identifier of Natural Products (AntiBase Library + ChemWindow Software)) (Renouvellement d’abonnement annuel) |
|
978EALDB04195 |
Wiley Identifier of Natural Products (AntiBase Library) (Abonnement d’un an)* |
|
978EALDB05512 |
Wiley Identifier of Natural Products (AntiBase Library) (Renouvellement d’abonnement annuel)* |
*Requiert le logiciel KnowItAll